
Noel Research Group
@noelgroupuva
Our research focuses on #FlowChemistry, #Engineering, #Synthetic #OrganicChemistry & catalytic methodology development. Headed by @tnoel82. @UvA_Amsterdam
ID: 3432425985
http://www.NoelResearchGroup.com 20-08-2015 06:38:45
3,3K Tweet
6,6K Followers
1,1K Following



Discover our new paper on photocatalytic activation of volatile alkanes with SO2 to create valuable sulfinates, key building blocks in many applications. The paper also shows the power of #FlowChemistry to handle gaseous reactants 🦸 Now Nature Communications: nature.com/articles/s4146…


Grateful to Organic Process Research & Development to highlight our work! For the original paper, just follow this link, it is open access: nature.com/articles/s4146… well done Ting…万婷, Luca Capaldo, Jonas, Felix de Zwart , Bas de Bruin, Angela and Timothy Noël

Rapid and scalable photocatalytic C(sp2)–C(sp3) Suzuki−Miyaura cross-coupling of aryl bromides with alkyl boranes (Bas de Bruin, Timothy Noël - Noel Research Group): nature.com/articles/s4146… (Nature Communications).


Check out our new paper on ligated boryl radicals as photocatalytic XAT mediators, enabling C(sp3)‒C(sp2) bond formation to yield valuable vinyl chlorides with excellent E/Z diastereoselectivity. Out now in Chemical Science: pubs.rsc.org/en/content/art… #photocatalysis #LBR #boron


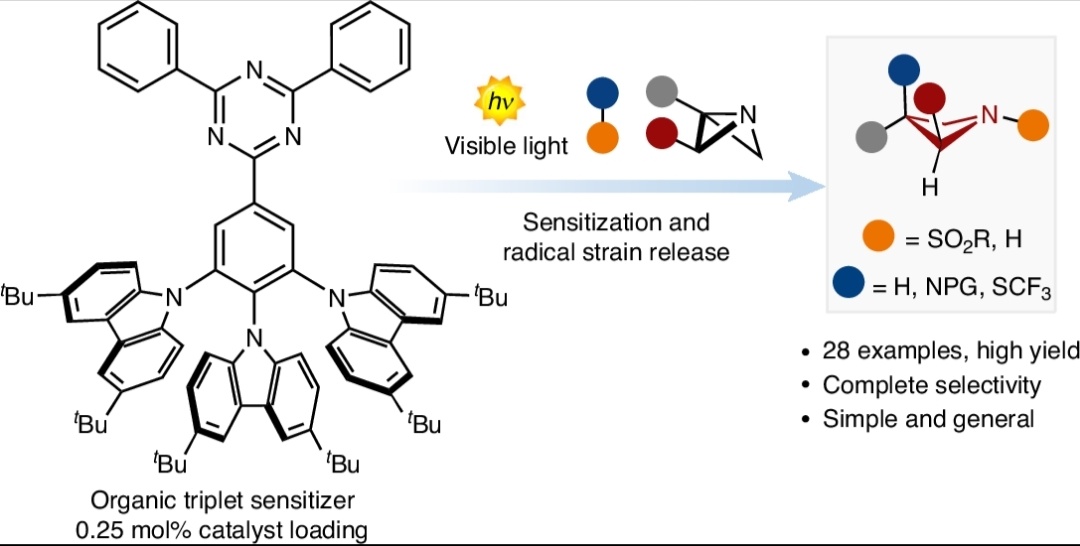
We're celebrating 🎉🎉 !! Our most recent work related to radical strain release photocatalysis for the synthesis of azetidines is now available Nature Catalysis in open access!!! 🔓🔓 A humongous job by all the team! 🐸nature.com/articles/s4192…🐸

Visible light-induced halogen-atom transfer by N-heterocyclic carbene-ligated boryl radicals for diastereoselective C(sp3)–C(sp2) bond formation by Noel Research Group and co-workers at the U. Amsterdam Chemical Science pubs.rsc.org/en/content/art…

Visible light-induced halogen-atom transfer by N-heterocyclic carbene-ligated boryl radicals for diastereoselective C(sp3)–C(sp2) bond formation (Chemical Science): pubs.rsc.org/en/content/art… (Noel Research Group, Timothy Noël).




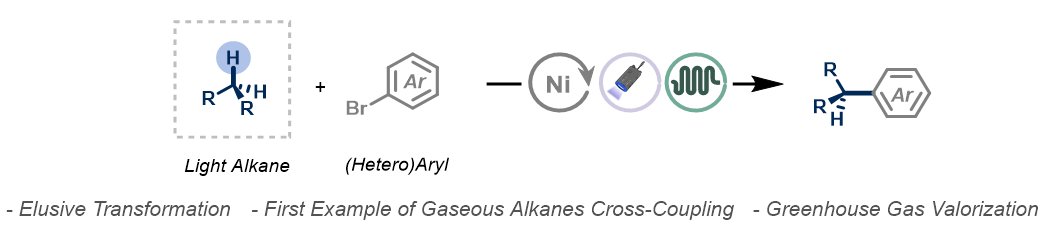
Alkylating Ar-Br with light alkanes? Previously thought impossible. Now, it's feasible using a combination of #flowchemistry, #HATphotocatalysis and #nickelcatalysis! 🔗Angewandte Chemie: onlinelibrary.wiley.com/doi/10.1002/an… Great collaborative effort, with DFT insights from Trevor A. Hamlin!

Feeling incredibly honored to receive the SCI Chimica Organica Junior Award "Organic Chemistry in its Methodological Aspects"! 🙏🤩🥳 Huge thanks to everyone who has supported me and believed in me. Grateful beyond words! 🫶 #Award #SCI2024


What a lovely surprise it is to find our work highlighted in my favourite journal 🤩 Thanks Organic Process Research & Development ! You can find more at pubs.acs.org/doi/full/10.10…


#ProcterPOW: Check out two effective flow strategies reported by Noel Research Group - the alkylation of aryl bromides using gaseous alkanes💡 and the environmentally friendly generation of reactive NCF3(PG), SCF3, and OCF3 anions for the introduction of heteroatom-CF3 motifs👀👇


