Kelly Norsworthy, MD
@drknorsworthy
Leukemia doctor #FDA & #JohnsHopkins. Deputy Division Director, Division of Hematologic Malignancies I. Opinions mine. Retweets not endorsed. COI: none.
ID: 1061077807432908800
10-11-2018 02:07:17
371 Tweet
980 Followers
1,1K Following



All set for the #ASH23 FDA Oncology session on benefit risk in new drug approvals. Come join us in Marriott Marquis Grand Ballroom 5-6! Cara Rabik, MD, PhD


FDA approvals in 2023: biomarker-positive subsets, equipoise and verification of benefit - via NatureRevClinOncol by Kelly Norsworthy, MD Rosa Lee-Alonzo & Rick Pazdur nature.com/articles/s4157… #OCEPublications


Highly recommended to apply! A great chance to meet FDA reviewers ASH | FDA Collaboration: A Workshop on Regulatory Science in Hematology ASH FDA Oncology Jennifer Gao Kelly Norsworthy Kelly Norsworthy, MD Greg Talal Hilal 🩺 hematology.org/advocacy/get-i…


FDA's Oncology Center of Excellence has a new project launching soon... and you are invited to take part! Check back on here on May 5, Cinco de Mayo, to find out. Kelly Norsworthy, MD Jennifer Gao Donna Rivera Steven C. Cunningham


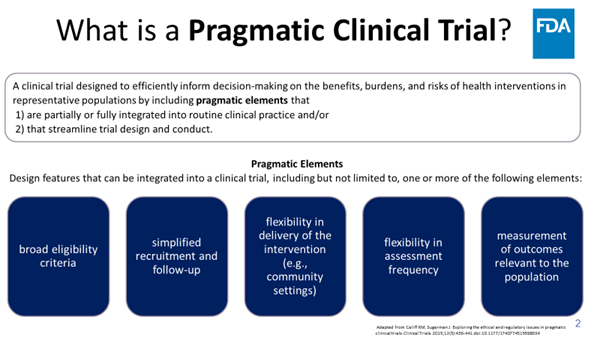
Prag-mat-ic: dealing with things sensibly and realistically in a way that is based on practical rather than theoretical considerations. (Oxford) FDA Oncology has a new project launching soon... Check back tomorrow! Kelly Norsworthy, MD Jennifer Gao Donna Rivera Steven C. Cunningham


Please consider posting your ideas for pragmatic clinical trials to this FDA Oncology crowdsourcing initiative #OCEProject5in5 starting today on 5/5 (no coincidence)!! Rick Pazdur Jennifer Gao Donna Rivera Steven C. Cunningham


Complete Remission with Partial Hematological Recovery as a Palliative Endpoint for Treatment of Acute Myeloid Leukemia - via Blood Journal. First author Robert Le, last author R. Angelo De Claro. sciencedirect.com/science/articl… #OCEPublications






Last chance to submit your #PragmaticTrials ideas to FDA Oncology! Or simply log in to the crowdsourcing platform to see ideas others have posed - comment, like, and pick your favorites!

An expert panel convened by FDA provides perspectives on drug development for the treatment of chronic myeloid leukemia in pregnant patients and patients who are breastfeeding - via Clinical Cancer Research. First author Jorge Cortes MD, last author Kelly Norsworthy, MD. aacrjournals.org/clincancerres/…

Reporting results of an expert panel on drug development for CML in pregnancy, coordinated with FDA Oncology Kelly Norsworthy, MD Georgia Cancer Center Clinical Cancer Research aacrjournals.org/clincancerres/…

Just published! With Kelly Norsworthy, MD and other FDA authors Ashley Woods, R. Angelo de Claro, Marc R. Theoret and E. Dianne Pulte. #OCEPublications

Hot off the press in The Oncologist,our paper on treatment of #AML in the community setting, I had the privilege of collaborating with outstanding group of oncologists and our colleagues at the FDA Oncology Kelly Norsworthy, MD academic.oup.com/oncolo/advance…