Margaret Van Meter, MD
@mvanmetermd
Medical oncologist @Intermountain, Director of Breast Oncology. Clinical research, quality improvement, cardio-oncology, provider wellness. All tweets my own.
ID: 1003286754382811136
03-06-2018 14:46:17
785 Tweet
778 Takipçi
442 Takip Edilen


EA4112: optimizing local therapy with DCIS with MRI and gene expression assay Patients with low risk DCIS score and no radiation had 5 year ipsilateral breast event rate ~5% Most applicable to patients >50yo with DCIS <2.5 cm ECOG-ACRIN Cancer Research Group Seema Khan of Lurie Cancer Center #SABCS23 #bcsm


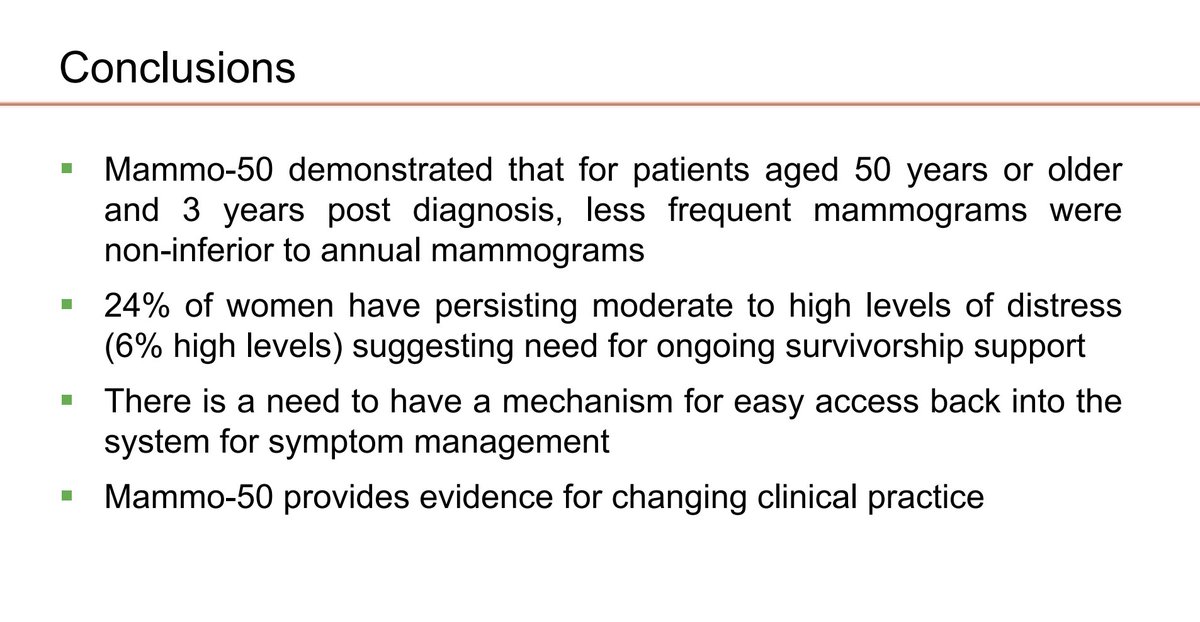
Mammo 50 trial At median follow-up of 8.7 years post-surgery, less frequent mammograms starting after 3 years post-surgery had noninferior RFI, BCSS, OS. High baseline distress at baseline and over time. Janet Dunn Warwick CTU #SABCS23 #bcsm

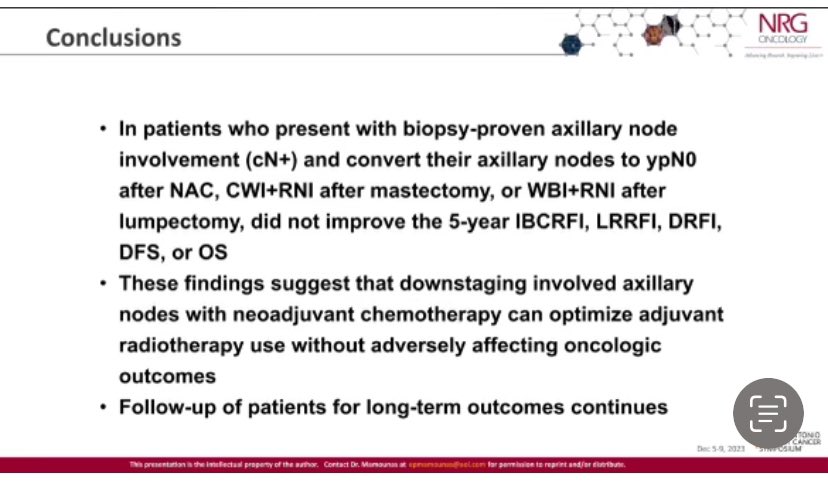
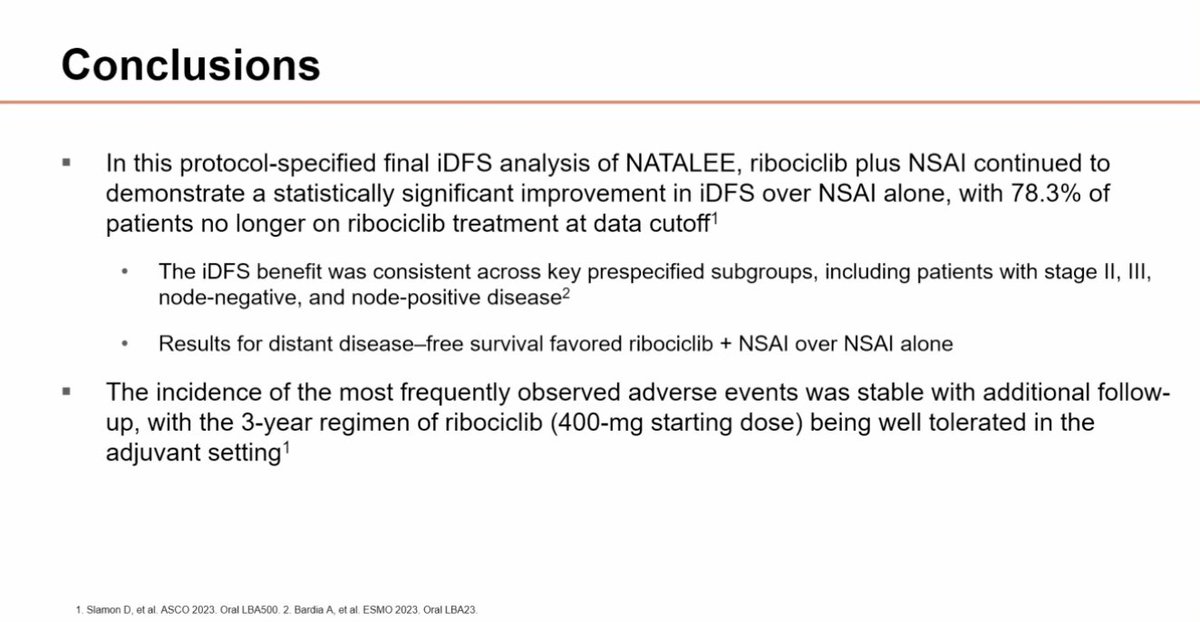
NATALEE: final iDFS analysis Ribociclib 400 mg 3 weeks on/1 week off x 3 years + NSAI in stage IIA-III, vs NSAI alone iDFS HR 0.75, 3.1% absolute benefit Benefit across subgroups, including stage II and N0 Distant DFS HR 0.75 OS immature MD Anderson Cancer Center #SABCS23 #bcsm

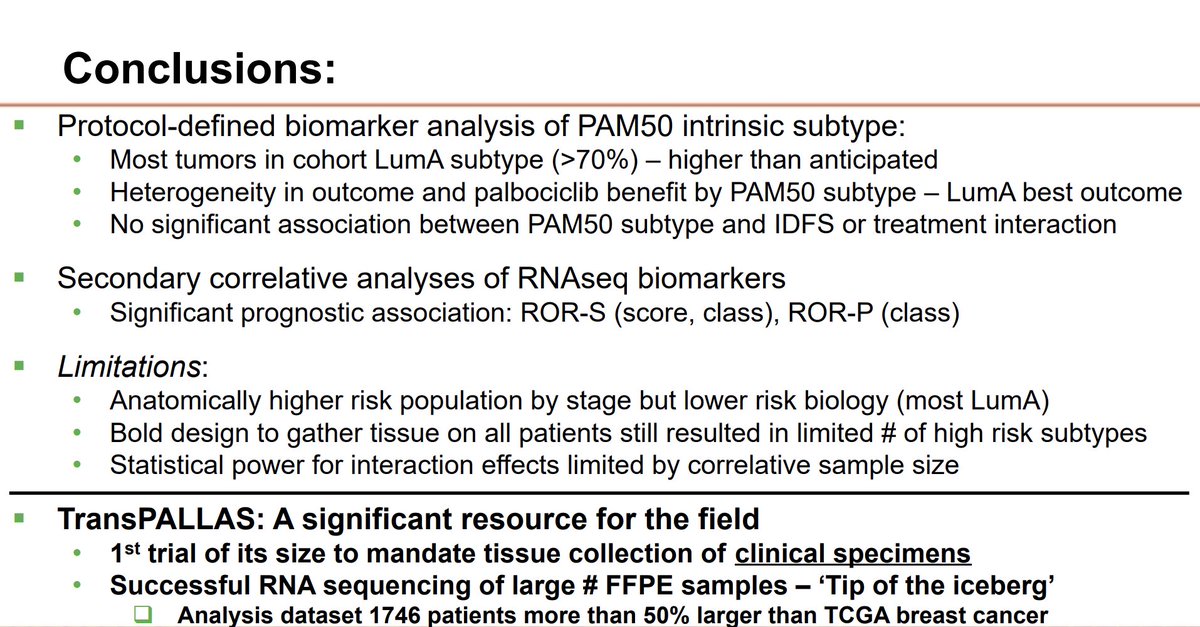
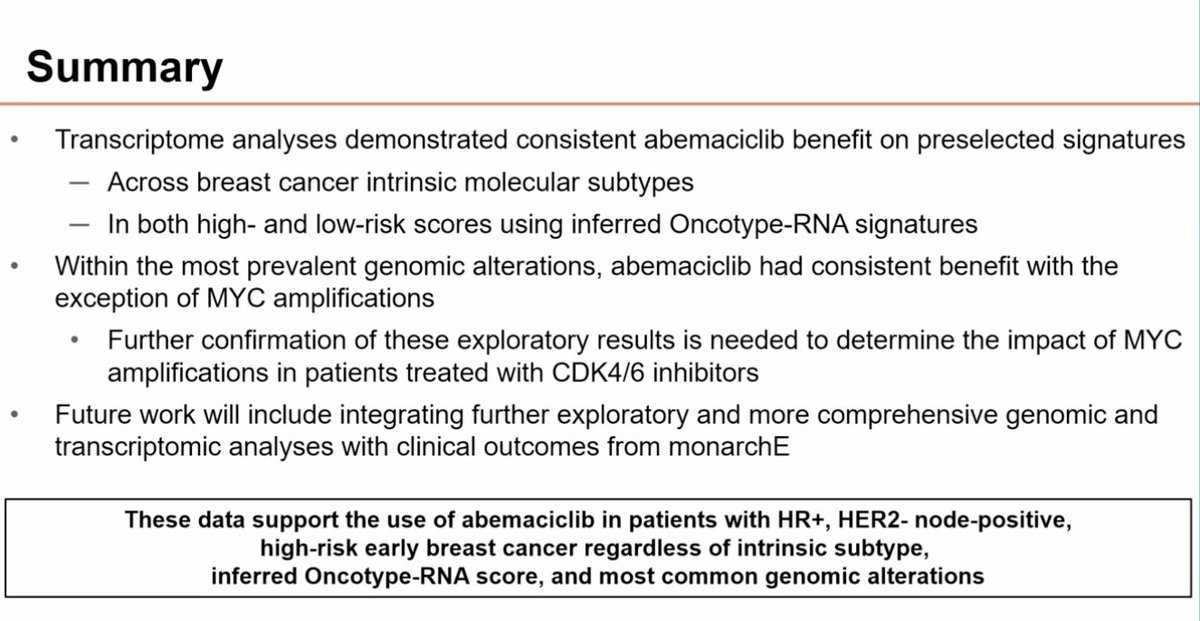
monarchE genomic & transcriptomic profiling Abemaciclib benefit seen across intrinsic subtypes and for inferred Oncotype RNA score both <=25 and >25 Possible diminished benefit in cancers with MYC amplification - exploratory Nicholas Turner The Royal Marsden NHS Foundation Trust #SABCS23 #bcsm


KATHERINE final iDFS and updated OS T-DM vs trastuzumab for residual invasive disease after neoadjuvant therapy Median follow-up 8.4 yr iDFS 80.8% vs 67.1%, HR 0.54 OS 89.1% vs 84.4%, HR 0.66 Benefit across subgroups Sibylle Loibl German Breast Group NSABP Foundation #SABCS23 #bcsm


INAVO120: Inavolisib vs placebo + palbociclib + fulvestrant in PIK3CA mutated HR+ HER2- advanced breast cancer progressed within 12 months adjuvant endocrine therapy mPFS 15 mo vs 7.3 mo 18-mo PFS 46% vs 21% median OS NE vs 31 mo Komal Jhaveri Memorial Sloan Kettering Cancer Center #SABCS23 #bcsm



Excellent plenary session comprehensive review of advances in TNBC by Melinda Telli, M.D. Stanford Cancer Institute #SABCS23 #bcsm


KEYNOTE-522: updated EFS Neoadjuvant pembro + chemo followed by adjuvant pembro, vs neoadjuvant chemo alone Median follow-up 63.1 mo EFS 81% vs 72% Benefit seen across disease stage, nodal status, and pCR outcome Peter Schmid of Barts Cancer Inst. #SABCS22 #bcsm


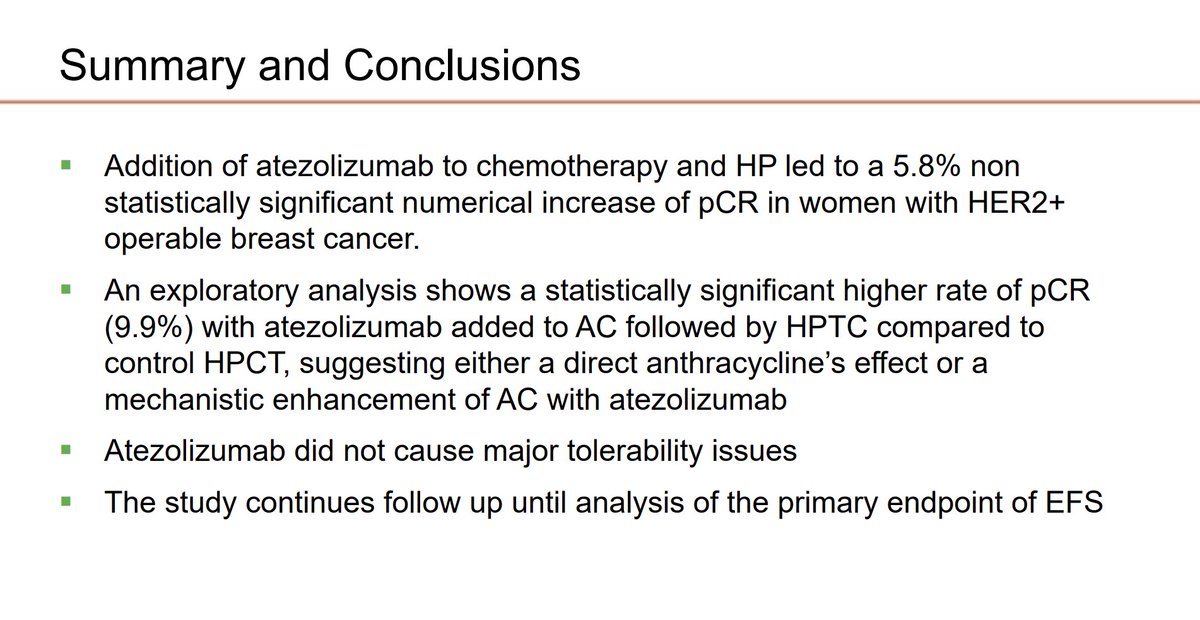
APTneo Michaelangelo: pCR in neoadjuvant chemo + trastuzumab/pertuzumab +/- atezolizumab in HER+ early breast cancer 5.8% increased pCR, not statistically significant Luca Gianni The Breast Cancer Research Foundation #SABCS23 #bcsm

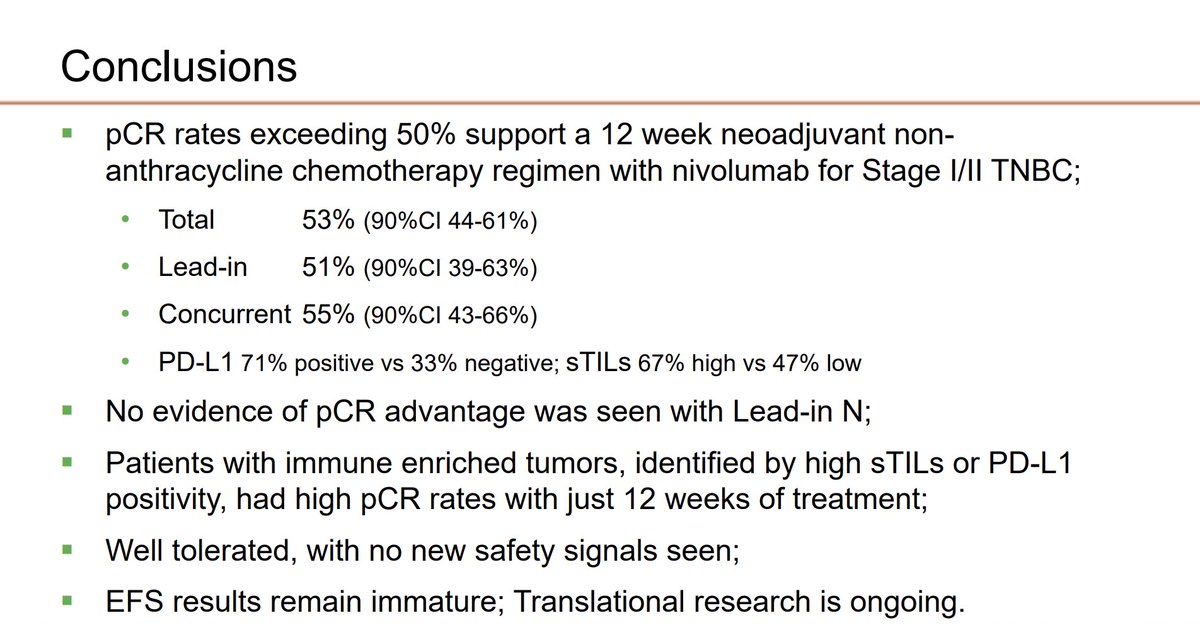
Encouraging phase II data on non-anthracycline chemo+nivolumab regimen in stages I and II TNBC BreastCancerTrials Nicholas Zdenkowski University of Newcastle #SABCS23 #bcsm


AACR Outstanding Investigator Award goes to Alana Welm, a leading scientist powering advances in breast cancer treatment. Many thanks to you and your team for your fabulous work! Alana Welm Huntsman Cancer Institute University of Utah The Breast Cancer Research Foundation #SABCS23 #bcsm